The question “Is water wet?” sounds like a simple question and yet has led to extensive debate among scientists, students, and many of us regular folks. At first glance, this question may seem straightforward, and you might be inclined to say that water is a liquid we always associate with being wet. Seems like a simple question, but this brings the definition of “wet” and some fundamental properties of water into the discussion.
Defining “Wet”
To explore whether water is wet, we need to define what “wet” means. In common usage, “wet” refers to a state where a liquid covers or saturates a surface, making it damp or moist to the touch. For example, when water contacts an object like a cloth or a hand, we say the object is wet. However, using the term “wet” to describe a liquid itself can be tricky since “wetness” typically describes how a substance interacts with something else. Wetness is characterized by the contact between a liquid and a solid, where the liquid displaces the solid-gas interface with a solid-liquid interface. The degree of wetting is influenced by adhesive forces between the liquid and the surface, as well as cohesive forces within the liquid itself (Cappelli et. al., 2015).
Scientific Perspective
Water has unique molecular properties that influence its interactions with other materials. Understanding these properties helps clarify the debate on whether water itself is wet:

- Molecular Structure of Water: Water molecules consist of two hydrogen atoms bonded to one oxygen atom (H2O). These molecules exhibit a strong attraction to each other, known as cohesion, which allows water droplets to form.
- Cohesion and Adhesion: Cohesion refers to the attraction between water molecules, while adhesion refers to water’s attraction to other surfaces. When water adheres to a material, it causes the sensation of wetness.
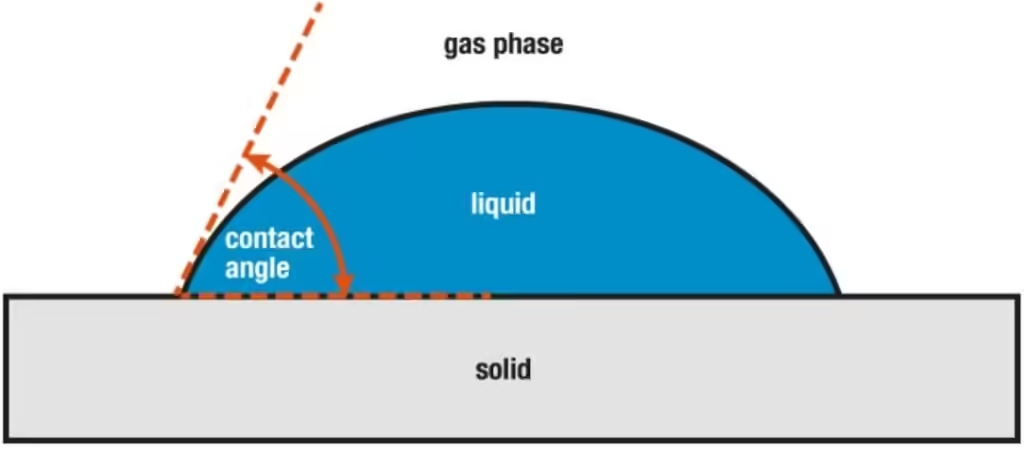
Adhesive Forces
It is the attractive force between unlike substances, such as mechanical forces (sticking together) and electrostatic forces (attraction due to opposing charges). In the case of liquids, the adhesion causes the liquid to cling to the surface on which it rests. When you pour water on clean glass, it tends to spread, forming a thin, uniform film over the surface. This is because the adhesive forces between the water and glass are strong enough to pull the water molecules out of their spherical formation and hold them against the surface of the glass, avoiding the repulsive forces between the molecules. Strong adhesive forces lead to lower contact angles (θ < 90°), resulting in complete wetting, while weaker forces yield higher contact angles (θ > 90°), causing water to bead up (Remsing, 2019).
Cohesive Forces
This term is a generic term that is a combination of intermolecular forces (i.e., hydrogen bonding and van der Waals forces) responsible for the bulk property of the liquids which resists separation. This type of attractive force exists between molecules of the same substance. To make it simple, rain falls in the form of droplets, rather than a fine mist, it is because of the strong cohesion of water molecules, which pulls its molecules together, forming droplets. This force tends to unite molecules of a liquid, gathering them together into a large cluster. When cohesive forces dominate over adhesive forces, water tends to form droplets rather than spreading out (Remsing, 2019).
Surface Tension
Water’s cohesive properties give it a high surface tension, meaning water molecules at the surface are tightly bound together. This tension allows water to form droplets and creates a boundary that can feel “wet” when encountered. In essence, water interacts with other surfaces to make them wet, but the question remains whether water in its pure state can be considered “wet.”
The Debate: Is Water Wet?
This question can be addressed from two different perspectives:
- Argument for Water Being Wet: Some argue that because water is a liquid, it must be wet by nature. When we immerse ourselves in water, we describe the sensation as “wetness,” leading some to conclude that water is inherently wet.
- Argument Against Water Being Wet: On the other hand, others argue that water itself isn’t wet. Wetness is a result of a liquid coming into contact with a solid surface. In this view, water causes other objects to become wet, but it cannot be wet on its own because wetness requires a comparison between a liquid and another material.
Both perspectives have merit, leading to the ongoing debate about whether water should be considered wet by itself.
Real-World Applications
Understanding the concept of wetness can be useful in various real-life situations:
- Cleaning and Washing: In cleaning processes, knowing how liquids interact with different surfaces helps us choose the right cleaning agents. Some surfaces may resist water, while others easily absorb it.
- Cooking: The wetness of food can affect its texture and cooking time. Water content in food plays a role in methods such as boiling, steaming, and frying.
- Scientific Research: In fields like physics and chemistry, understanding water’s properties can be important for studying surface tension, liquid-solid interactions, and fluid dynamics.
Common Misconceptions
The question about water’s wetness often arises from misunderstandings related to language and perception. Here are some common misconceptions:
- Wetness as an Intrinsic Property: Many assume wetness is an inherent quality of all liquids, but this isn’t entirely accurate. Wetness is the result of a liquid interacting with another surface. The mercury does not wet the glass but water wets. This is because the cohesive force is weaker than the adhesive force in water but the Cohesive force is stronger than the adhesive force in mercury. Adhesive force is weaker than cohesive force in water but the adhesive force is stronger than cohesive force in mercury.
- Confusion with State of Matter: People sometimes confuse wetness with the state of matter, believing that because water is a liquid, it must be wet. However, wetness specifically describes the effect of a liquid on a solid.
These misconceptions demonstrate how our language can influence our understanding of scientific concepts.
Conclusion
The question “Is water wet?” doesn’t have a straightforward answer, as it depends on how we define and perceive “wetness.” While water can make other surfaces wet, considering water itself as wet remains debatable. Ultimately, the discussion highlights the importance of critically analyzing everyday concepts and understanding the physical properties of substances.
FAQs
- What does “wet” mean?
Wetness describes a condition where a liquid covers or saturates a surface. - Is water considered wet by itself?
This is debated, but many argue that water causes wetness instead of being wet itself. - Can other liquids be considered wet?
Wetness generally applies to any liquid that covers a solid surface. - Why do people debate whether water is wet?
It’s a philosophical question that challenges our understanding of language and physical properties. - Is air wet?
Air itself isn’t wet, but it can contain water vapor or humidity. - What makes a solid wet?
A solid becomes wet when a liquid adheres to its surface. - Does temperature affect wetness?
Temperature can influence how liquids interact with surfaces, affecting wetness. - Can a gas be considered wet?
Gases aren’t wet, but they can carry moisture. - Is wetness a scientific term?
People commonly use “wetness” in everyday language, but it isn’t a precise scientific term. - Why does water feel wet?
The sensation of wetness comes from water’s adhesion to the skin and the body’s perception of moisture.
Thank you for reading, for more interesting articles visit our homepage.



